Enhancing institutional capacity of public authorities and stakeholders and efficient public administration.

MAIN OBJECTIVES OF THE PROJECT
The main objective of the project is to build inter-institutional collaboration capacity by encouraging public authorities and key stakeholders in the healthcare sector in Public Administration to create joint solutions for systems harmonization and efficient cross-border biobanking , through a common platform for the harmonization of biobanking and the creation of the first pilot cross-border biobank of biological samples.
The biobank is a collection of biological material and associated data intended for use in research and clinical care. Setting up harmonized and shared biobanks that comply with GDPR, national and EU legislation and ethical standards represents a common challenge in the program area (PA). In fact, the current absence hinders the management and exchange of biological samples necessary for standardized and quality research. The C3B project led to the development of a model for the development of integrated management biobanks in the program area
A biobank is a collection of biological material and associated data intended for use in research and clinical care. Setting up harmonized and shared biobanks that comply with GDPR, national and EU legislation and ethical standards represents a common challenge in the program area (PA). In fact, the current absence hinders the management and exchange of biological samples necessary for standardized and quality research. The overall objective of the project is to improve the capabilities of cross-border institutional collaboration by encouraging public authorities and key stakeholders in the biobanking sector to identify common solutions for the harmonization of processes and for more efficient cross-border management of biobanks and networks. biobanks. The objective will be achieved with the development of a common biobank model through an integrated cross-border management platform. This will be verified in a pilot action to set up a standardized biobank supported by the regional authorities. The target groups will be policy makers, public authorities, regulators, companies, healthcare systems, universities and research institutes and clusters.
ACTIVITY
Start of the C3B project
On October 1, 2021, the C3B project officially starts. During the first Kick-Off meeting, the project partners planned the activities for the following months, defining priorities and working groups, in line with the operational calendar (GANTT chart).

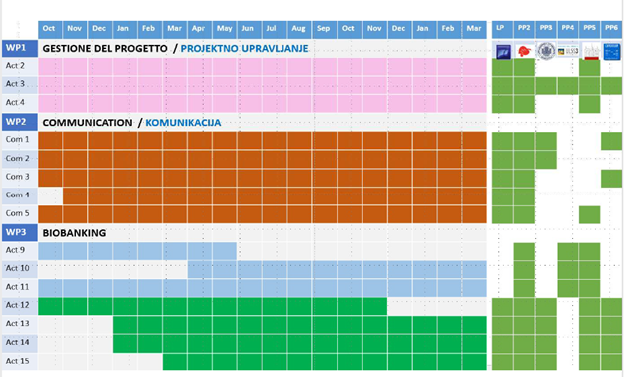
Gantt chart of planned project activities.
The C3B project is developed on the basis of the scheme of planned activities, some of which include:
Census of existing resources
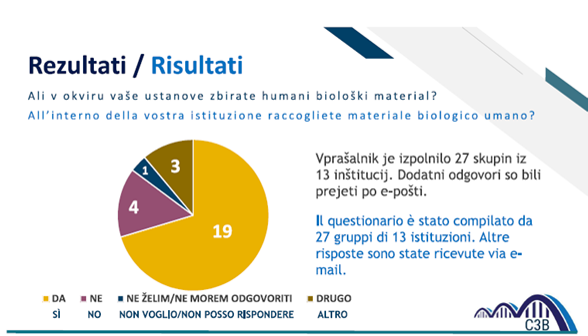
On 19 January 2021, the stakeholder involvement activity began, for example in a meeting held online, the project and purpose were presented to healthcare directors of hospitals, staff responsible for departments, university staff, placing emphasis on the challenges to be faced and needs in the biobanking sector

At the same time, a census activity of biobanks and collections of biological samples in the program area began, as well as the propensity to join and collaborate with a biobank
Definition of standardized and shared operating procedures
In order to regulate and standardize (harmonize) the guidelines that regulate the activities of biobanks in the Program AREA, the C3B project brought together a working group, made up of various representatives of the institutes participating in the project. The first phase of this activity concerned the acquisition of the guidelines already in force in the hospital centers of the program area, in order to identify the shared points and use them for the definition of new ones aligned with the current EU legislation, with the defined needs from the biobank and the activities of collection centers.
Creation of a standardized data management system
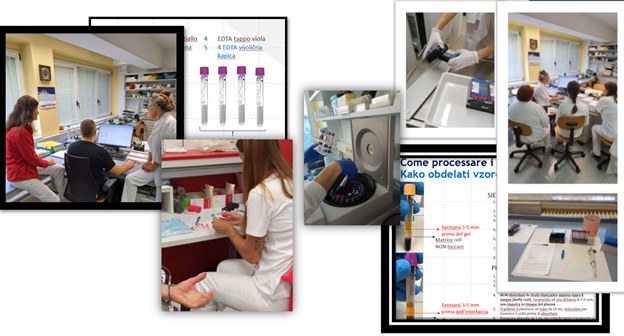
The usefulness of a biological sample for scientific research purposes is greater the more data associated with it. The working group defined both what the minimum essential information (IEM) is, necessary to guarantee the quality and usefulness of the sample, and a whole list of data relating to the pathologies in question that must accompany a sample. For example, for a blood sample derived from a subject with steatohepatitis, the data relating to the lipid profile, liver enzymes, etc. will be important. All this in a strictly anonymous format, i.e. without being able to trace the identity of the voluntary donor. Sample and data management software were evaluated during the project. A barcode and QR code reader was also acquired which allows each individual sample to be uniquely identified and monitored from the moment of collection to storage and use.

Training activity
The training activity implemented through seminars, workshops and practical activities was important throughout the duration of the project. The training involved the staff of the collection centers, i.e. hospital staff (doctors, nurses, laboratory staff) who were trained on how to enroll donors, on the protocols to follow, on the information regarding the samples that must be recorded, on the methods of handling the sample, on the safety procedures, and on the operations that guarantee the quality of the sample. The training also involved the staff of the research and sample storage centers (Biobanca). The professional figures involved include biologists, biochemists, laboratory technicians, doctors, etc. The staff has been trained on how to handle samples, receive and store them. Training was also carried out on the use of the newly acquired equipment as well as on all procedures relating to quality and safety control.
Feasibility and compliance tests
Evaluation of standardized and harmonized protocols: from sample collection to final application (use )
Once the SOPs (Standard Operating Procedures) were defined, ample space was dedicated to evaluating them in terms of feasibility, compliance, adaptability to the operating environment, quality of the samples and the data obtained. The derived reports allowed the implementation of the SOPs with some improvements regarding the processes and handling of biological samples
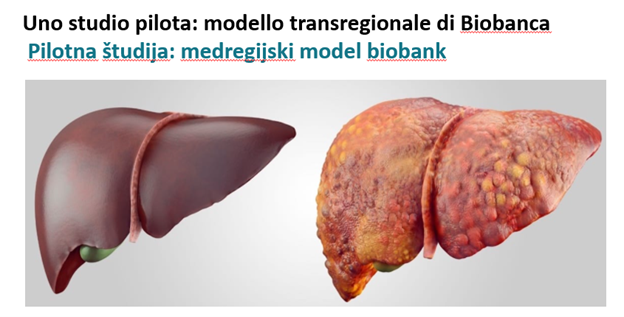
A pilot study: transregional Biobank model
With the C3B project it has started a pilot biobank in the program area divided into two storage hubs, one dedicated to the storage of samples donated by healthy subjects, mostly belonging to the Transfusion Center of the Republic of Slovenia and one dedicated to biological samples collected from donors with liver pathologies at the Italian Liver Foundation. At these hubs, spaces have been set up with new instrumentation, such as -80°C ultra-freezers and probes for remote temperature monitoring.
The launch of the biobank made it possible to implement the scientific research studies currently underway at the institutes involved in the project.
MAIN PROJECT OUTPUTS
Definition of shared Standard Operating Procedures
During the project, the harmonized Standard Operating Procedures for the management of biobanks and for the collection, manipulation and storage of biological samples (blood and derivatives, cells and tissues) were produced.
These documents constitute guidelines that regulate in detail all the steps necessary for the management of the infrastructure and samples


Launch of pilot biobank
In order to test the adequacy of the SOPs formulated for the cross-border reality, the C3B project has started a pilot biobank. The spaces have been set up at the Italian Liver Foundation (LP) and the Ljubljana Transfusion Center (PP2), and the necessary equipment has been acquired, including -80°C ultra-freezers and monitoring systems

Pilot Studies
The C3B project made it possible to implement and accelerate the scientific research studies underway at the institutes involved, as well as to develop new ones. Most studies have focused on the identification of bio -markers of liver diseases present in blood or derivatives. The results of some studies have been published in international scientific journals.
LIST OF SCIENTIFIC PUBLICATIONS:
|
PP involved |
Authors |
Title |
Sector |
Tongue |
Result / Use |
Availability |
|
LP-PP3 |
Johanna Matilainen , Pablo Giraudi, Silvia Palmisano, Claudio Tiribelli, Natalia Rosso. |
EXTRACELLULAR VESICULES AND LIQUID BIOPSY: NEW GENERATION OF BIOMARKERS IN THE FIELD OF PRECISION MEDICINE FOR LIVER DISEASE |
Hepatology, biomedical research |
ENG |
The cross-talk between adipose tissue and liver mediated by Extracellular Vesicles could act as a potential diagnostic tool but also an innovative therapeutic target for NAFLD. |
public |
|
LP |
Grisetti L, Võ NVT 3, Nguy ễ n NNQ, Crocè LS, Visintin A. Tiribelli C, Pascut D. |
MiR-3201 as a Prognostic Blood Biomarker for Curative Treatments in Hepatocellular Carcinoma |
Hepatology, biomedical research |
ENG |
Identification of circulating biomarkers with prognostic value for liver cancer |
public |
|
LP and PP3 |
Noel Salvoza, Pablo Giraudi, Silvia Palmisano, Claudio Tiribelli, Natalia Rosso. |
The potential role of Omentin-1 in Obesity-Related Metabolic Associated Fatty Liver Disease (MAFLD): Evidence from translational studies
|
Hepatology, biomedical research |
ENG |
Reduced levels of omentin-1 contribute to the development of MAFLD. Omentin-1 supplement reduces inflammation, ER stress, and oxidative stress likely through inhibition of the NF- kB pathway and may also play a role in regulating glucose and insulin metabolism |
public |
|
LP and PP3 |
Melissa M. Milito, Deborah Bonazza, Claudio Tiribelli, Silvia Palmisano, Natalia Rosso, and Pablo J. Giraudi |
Hypoxanthine plasma level as a potential non-invasive biomarker of metabolic associated steatohepatitis
|
Hepatology, biomedical research |
ENG |
Plasma hypoxanthine levels in patients with MAFLD could be used as a minimally invasive biomarker for the identification of patients with MASH. |
public |
|
LP and PP3 |
Allen A. Laraño, Silvia Gazzin, Deborah Bonazza, Silvia Palmisano, Claudio Tiribelli, Natalia Rosso and Pablo J. Giraudi
|
In silico identification and validation of biomarkers for metabolic associated fatty liver disease in an in vivo mouse model and severely obese subjects
|
Hepatology, biomedical research |
ENG |
Plastin-1 is overexpressed in both murine and human livers during the development of MAFLD. |
public |
|
LP and PP4 |
Barbaglia M, Dittadi R, Fabricio ASC, Gion M, Pascut D, Tiribelli, C Trevisiol C. et., al |
Comparative synoptic analysis of the guidelines to define the appropriate use of circulating biomarkers in the diagnosis of hepatocellular carcinoma |
Hepatology, biomedical research |
ENG/ITA |
Consensus identification of biomarker use for the diagnosis of liver cancer |
Publish |
|
LP and PP4 |
Dittadi R, Fabricio ASC, Gion M, Pascut D, Tiribelli et., al |
The Public's Awareness of and Attitude Toward Research Biobanks – A Regional Survey |
Biobanks |
ENG/ITA |
Citizens' propensity towards donating biological samples for research/citizen awareness |
public |
|
PP2, PP5 |
Režen T., Nahtigal K., Čurin Šerbec V., Černilec M., Gracar M., Šprohar M., Lampreht N., Stanišić S. |
Analysis of biobanks and the need for biological samples in western Slovenia. |
biobanks |
SLO |
Evaluation of the regulation of the biobanking sector in Western Slovenia and its need assessment of samples. The results are useful for planning pilot biobanking of healthy donors and regulating the situation in the field of biobanking. |
Publish Electronic format |
Memoranda of understanding
The C3B project culminated with the signing of a Cross-Border Agreement , i.e. between Institutes located in the Program Area, for a common effort in harmonized collaboration in sample collection, sharing of good practices and solutions relating to Biobanking . This document was signed at the end of March 2023 between the Italian Liver Foundation , Zavod Republike Slovenije za transfuzijsko medicinano and the University of Ljubljana and will guarantee close collaboration between the institutes in the future in promoting the development of Biobanks in the territory and at the same time the scientific studies associated with them.
COMMUNICATION STRATEGIES
With the C3B project the aim was to raise awareness among various target groups in the Program Area on the benefits derived from the presence of biobanks, understood as essential service infrastructures for scientific research and technological progress, in the territory.
This strategy involved the organization of events in online and in-person formats, including seminars, workshops and conferences. It involved the production of information material, the dissemination of activities and their results through radio and television broadcasts, the press and social networks ( facebook , linkedin , youtube )
These activities have gradually been adapted to different target groups such as healthcare workers, the general public, companies in the health sector, universities and research centres, patient associations

PROJECT EVENTS
06/06/2022 WORKSHOP
Center for Functional Genomics and Bio -Chips CFGBC Symposium
Organized by Univerza v Ljubljani , Medicinska fakulteta (Online and in person)
Followed by 89 people
http://cfgbc.mf.uni-lj.si/c3b/
09/20/2022 WORKSHOP
Biobanks for scientific research: the Udine hub, the experiences in Friuli and successful European models
Organized by the University of Udine
Followed by 40 people
Program
https://qui.uniud.it/documents/ 4302/BIOBANCHE_Programma.pdf
Press release
https://qui.uniud.it/ricerca-e-associazione/universita-di-udine-asufc-e-cro-di-aviano-insieme-per-la-prima-biobanca-in -Udine-pole-network/
On 20 September 2022, a workshop entitled "Biobanks for scientific research: the Udine hub, the experiences in Friuli and successful European models" was held, organized by the University of Udine. This event offered an important opportunity for discussion and in-depth analysis on the role of biobanks in scientific research, with a specific focus on the local context of Friuli and on examples of excellence at a European level.
The workshop provided a platform for the exchange of knowledge and experiences between participants, promoting a constructive dialogue on the challenges and opportunities related to biobank management. Furthermore, he highlighted the importance of international and cross-border cooperation to advance biomedical research and improve public health.
Youtube video
https://www.youtube.com/watch?v=61jQfpB06VU&list=PLZXdmAb9TWmPJitAmZYF8VDLGTU7agg6M&index=31
09/29/2022 WORKSHOP
Presentation of Project Objectives and Results
Organized by Zavod Republiclike Slovenije za transfuzijsko medicinano (ZTM) and University of Ljubljana
Followed by 39 people
http://2014-2020.ita-slo.eu/it / tutte-le-notizie/events/c3b-evento-di-presentazione-obiettivi-e-risultati
10/12/2022 ONLINE WORKSHOP
C3B -Cross-border pilot projects to boost innovation in the health system through data sharing
Organized by European Community Brussels
Followed by 71 people
EU website
site https://regions-and-cities.europa.eu/eu-cohesion-policy-pushes-shared-and-digitally-connected-health-system
Interreg website
http://2014-2020.ita-slo.eu/it/tutte-le-notizie/events/c3b-cross-border-pilot-projects-boost-innovation-health-syste m -throu g h-data
10/20/2022 SEMINAR
Cross-border project for the efficient management of biobanks, C3B
As part of Kemomind Science Conference 2022 "Our DNA is Our Identity: from Genome Editing, Biobanking, and Cell Biology"
Organized by Zavod Republiclike Slovenije za transfuzijsko medicine (ZTM)
Followed by 50 people
Program
h ttps://www.kemomind.com/ke m omind-science-conference-2022
Journal
https://www.kemomind.com/_files/ugd/6f172c_abe680dd9d2349789d9b63b97576e5 6 5.pd f
03/24/2023 C3B FINAL EVENT
Organized by the Italian Liver Foundation
Followed by 39 people
http://2014-2020.ita-slo.eu/it/tutte-le-notizie/events/save-date-evento-finale-progetto-c3b-24032023
The final c3b event was held in person on 24 March 2023 at Savoia Excelsior Palace Trieste
VIDEO
The C3B Project has created a series of 7 videos with the aim of raising public awareness and spreading knowledge about the concept of biobanking. Biobanks are facilities that collect, conserve and manage human biological samples (such as blood, tissues, DNA) intended for scientific research. These samples are crucial for studying diseases, developing new treatments and personalized medicine.
The videos produced within the C3B Project aim to:
1. Educate the public on the importance of biobanks for medical and scientific progress.
2. Explain how biological samples are collected and stored .
3. Illustrate the potential applications of data and samples stored in biobanks in medical research.
4. Raise awareness of the need for informed and conscious participation by sample donors.
5. Promote transparency and trust in the biobank management process.
These videos represent an important tool to bring the community closer to science, facilitate understanding of the procedures involved in biobanking and encourage greater participation and support from the public.
C3B video playlist:
https://www.youtube.com/playlist?list=PLgc96DD7WLFOjfE_RYHkjxUI02lyzlbMX
C3B Interreg ITA-SLO - What is a biobank? Why are you biobanking ? What is a biobank?
https://www.youtube.com/watch?v=tbBE95c5Zok&list=PLgc96DD7WLFOjfE_RYHkjxUI02lyzlbMX&index=7
ITA
C3B Interreg ITA SLO - Cross-border platform for efficient management of biobanks
https://youtu.be/fxfi3e0MCSs?si=AifnGSmFVmVVKr3I
Discover the Italian Liver Foundation FIF Onlus - Lead Partner of the C3B Interreg ITA-SLO Project
https://youtu.be/7x-tGVUWPWM?si=sA9tGfClkjg0gvT5
SLO
C3B Interreg ITA SLO – Čezmejna platforma za učinkovito upravljanje biobank
https://youtu.be/AwajQMolkHg?si=jU-zRK2gNkwkCr0V
Odkrijte Italijanska Fundacija za Jetra , vodilni partner project C3B Interreg Italian
https://youtu.be/Q7RgUaGYNys?si=yRROTqtUDYuKzGU9
ENG
C3B Interreg ITA SLO - Crossborder platform for efficient management of biobanks
https://youtu.be/AwajQMolkHg?si=jU-zRK2gNkwkCr0V
Discover the Italian Liver Foundation - Lead Partner of the Interreg ITA-SLO C3B Project
Lead Partner

Project partner 1

Project partner 2

Project partner 3
Project partner 4

Project partner 5

| EASL-SLD-2023-Abstract-book-FULL_pag47-48.pdf ( 205 bytes, published on 23 May, 2024 - 11:38 ) | |
| EASL-SLD-2023-Abstract-book-FULL_pag51.pdf ( 18 bytes, published on 23 May, 2024 - 11:38 ) | |
| Uni-LJ.si_17.02.2022_Eng.pdf ( 543 bytes, published on 23 May, 2024 - 11:38 ) | |
| Kemomind Book of abstracts 2022 - FINAL ALL - Objava splet-1.pdf ( 17 bytes, published on 23 May, 2024 - 11:38 ) | |
| MiR-3201 as a Prognostic Blood Biomarker_2022.pdf ( 1 byte, published on 23 May, 2024 - 11:38 ) | |
| The potential role of Omentin-1 in Obesity-Related MAFLD_Evidence from translational studies.pdf ( 2 bytes, published on 23 May, 2024 - 11:38 ) | |
| C3BProgram srečanja 29. sept 2022W.pdf ( 146 bytes, published on 23 May, 2024 - 11:38 ) | |
| leaflet_C3B_def_ENG.pdf ( 1 byte, published on 23 May, 2024 - 11:38 ) | |
| POSTER C3B.pdf ( 415 bytes, published on 23 May, 2024 - 11:38 ) | |
| C3B RollUp (80 × 200 cm).pdf ( 6 bytes, published on 23 May, 2024 - 11:38 ) | |
| Evento finale_Zaključni dogodek_C3B_FINAL.pdf ( 373 bytes, published on 23 May, 2024 - 11:38 ) | |
| C3B evento finale_Presentation.pdf ( 4 bytes, published on 27 May, 2024 - 13:52 ) | |
| Presentazione EWRC_European_Week_Regions_Cities.pdf ( 4 bytes, published on 27 May, 2024 - 13:52 ) |
KEY PROJECT OBJECTIVES
The main objective of the project is to build inter-institutional collaboration capacity by encouraging public authorities and key stakeholders of the health care sector in the PA to create joint solutions for harmonized cross-border biobanking through a common platform and the creation of the first pilot cross-border biobank of biological samples
The biobank is a collection of biological material and associated data intended for research and clinical care purposes. Establishing harmonized and shared biobanks that comply with GDPR, national and EU legislation, and ethical standards represents a common challenge in the program area (PA). Indeed, the current absence hampers the management and exchange of biological samples necessary for standardized and high-quality research. The C3B project has led to the development of a model for the integrated management of biobanks in the program area.
CURRENT STATUS OF IMPLEMENTATION
The C3B project aimed to develop, through a network of cross-border inter-institutional collaboration, joint solutions and development plans for creating a harmonized biobank system in the PA. This was achieved by involving key stakeholders, including clinical centers, universities, and various other institutions. This materialized in the establishment of the first cross-border biobank for biological samples of liver diseases and biological samples donated by healthy volunteers.
Thanks to the cross-border collaboration between research centers, hospitals, and public administrations, the program area is becoming fertile ground for the development of biobanks in support of research activities. Through the establishment of extensive collaborative networks, shared standard operating procedures, guidelines, and cross-border agreements, the C3B project has initiated a process of harmonization and simultaneous development of biobanks in the PA, launching the first hepatological biobank flaked by a biobank dedicated to the collection of samples donated by healthy volunteers